Calculate the nuclear binding energy for 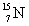 using the following information: proton mass = 1.0073 g/mol
using the following information: proton mass = 1.0073 g/mol
Neutron mass = 1.0087 g/mol
Electron mass = 0.00055 g/mol 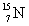 = 15.0001 g/mol
= 15.0001 g/mol
1) 0 g = 9.0 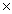 1013 J
1013 J
A) 1.3 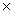 1011 J/mol
1011 J/mol
B) 9.0 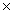 109 J/mol
109 J/mol
C) 1.1 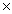 1013 J/mol
1013 J/mol
D) 8.1 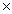 1013 J/mol
1013 J/mol
Correct Answer:
Verified
Q2: Gamma radiation has
A)a mass of 4 amu.
B)a
Q3: As the pressure exerted on a gaseous
Q4: Which is not affected as it passes
Q5: A beta particle has
A)a mass of 4
Q6: The curie,which is the unit used to
Q8: An alpha particle has
A)a mass of 2
Q9: As the pressure exerted on a gaseous
Q10: As the temperature of a solid radioisotope
Q11: All nuclides of which element must be
Q12: The mass of an alpha particle is
A)1
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents