A neutral isotope of an element contains 21 electrons and 24 neutrons.What is the following representation for this nucleus?
A) 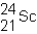
B) 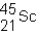
C) 
D) 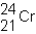
Correct Answer:
Verified
Q42: Atoms that have the same atomic number
Q43: The weight % of S in K2SO4
Q44: Write the formula for a compound consisting
Q45: Determine the number of electrons and protons
Q45: How many electrons are found around the
Q46: Consider the representation shown below.It should be
Q49: What is the molar mass of Ba3(PO4)2?
A)232.30
Q53: An isotope of a given element has
Q59: What is the number of moles of
Q76: Oxalic acid is found in many plants,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents