An isotope of gallium consisting of 31 protons and 37 neutrons can be represented using the symbol shown below. 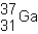
Correct Answer:
Verified
Q69: Isotopes of the same element always have
Q83: One mole of any substance will contain
Q84: The pure substance, water, contains both hydrogen
Q85: A scanning tunneling microscope (STM)relies on a
Q87: Protons and neutrons have approximately the same
Q89: The atomic mass number is a whole
Q93: Neutral isotopes of the same element have
Q94: Calcium supplements can be taken in 1,000
Q95: In naturally occurring samples, all elements exist
Q96: An MRI instrument cause the hydrogens in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents