Which of the following molecular geometries would most likely give rise to a nonpolar compound?
A) 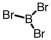
B) 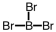
C) 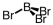
D) 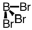
Correct Answer:
Verified
Q24: The shape of a BF3 molecule is
Q26: The correct formula of an ionic compound
Q27: The correct name for K2SO4 is _
Q30: What single condition is required to insure
Q31: Which of the following correctly illustrates hydrogen
Q31: Which of the following would be a
Q33: The correct name for KHPO4 is _
Q37: In describing the strength of interparticle forces,
Q38: The correct name for the covalent compound
Q39: A polar molecule must have
A)polar bonds.
B)an unsymmetrical
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents