In which of the following does an element have an oxidation number of +4?
A) TiO2
B) HBr
C) SO3
D) 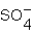
Correct Answer:
Verified
Q1: Consider the reactants and propose the
Q2: Single replacement reactions are always
A)redox reactions.
B)nonredox reactions.
C)combination
Q3: What is the oxidation number of Cl
Q6: What substance is reduced in the
Q8: Which species would be considered a
Q9: What is the oxidizing agent in
Q10: What is the oxidation number of Br
Q11: Propane,C3H8,burns to produce carbon dioxide and
Q14: Which of the following is an oxidizing
Q19: Which of the following contains the metal
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents