Citric acid,a natural food preservative,accounts for the tartness of citrus fruits.It is shown below.About 730 g of this material can be dissolved in water,making a liter of solution.However,only about 1.5% of it dissociates.As such,it would be considered a _____. 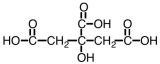
A) nonelectrolyte
B) strong electrolyte
C) pseudoelectrolyte
D) weak electrolyte
Correct Answer:
Verified
Q44: One test to determine if a mixture
Q45: Express the following concentration of solution in
Q47: Which of the following is not considered
Q49: You have a patient who is suffering
Q53: Which of the following would be considered
Q55: A solution is prepared at 75 °C
Q56: The ability to see the scattering of
Q57: Drinking water can be purified by which
Q58: An ion in solution that is surrounded
Q59: When a patient's blood electrolyte levels are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents