The following reaction is observed in a lab experiment: A + 2B C + D
In this experiment,it required 750 s for the concentration of C to change from 0.333 M to 0.750 M.What is the rate of the reaction?
A) 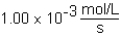
B) 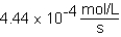
C) 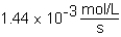
D) 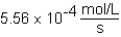
Correct Answer:
Verified
Q43: The device used to measure heat produced
Q44: Which of the following is NOT true
Q52: If we remove CO2 from the following
Q54: The equilibrium constants for a series of
Q56: A room filled with a 2:1 mixture
Q59: A patient complains about having painful muscle
Q59: Consider the following energy diagram.Which letter represents
Q60: Based upon the energy diagram given below,which
Q63: Butane, C4H10, burning in air, is an
Q76: Reaction rates are determined experimentally.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents