Phosphoric acid,H3PO4,undergoes three dissociation reactions.Which of the three acids is the weakest?
A) 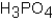
B) 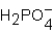
C) 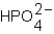
D) More than one response is correct.
Correct Answer:
Verified
Q3: Some salts isolated by evaporation retain water
Q22: Which salt shifts the pH when dissolved
Q25: A 25.00 mL sample of H2SO4 acid
Q26: Identify the substance with the lowest pH.
A)orange
Q28: Which of the following mixtures would represent
Q28: A higher pH corresponds to _ .
A)
Q30: A solution has a pH of 11.60.The
Q33: Which of the following salts would produce
Q35: When an acid is analyzed by adding
Q40: The classification of an acid or base
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents