The expression for the Ka of the weak acid HF would be which of the following?
A) 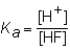
B) 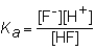
C) 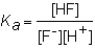
D) 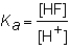
Correct Answer:
Verified
Q41: Which of the following sets of reactants
Q42: A patient comes to you suffering from
Q44: HCl will react with
A)BaO
B)CaCO3
C)Mg
D)All three are correct.
Q48: Sodium phosphate can be used as a
Q49: How many meq are contained in 25.00
Q50: What is the pKa of an acid
Q54: Which of the following would you expect
Q55: To determine the number of equivalents of
Q56: When a solution of HNO3 is added
Q59: Consider the following list of p K
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents