Anthracene has the structure given below and is an example of a polycyclic aromatic compound. 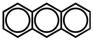
Correct Answer:
Verified
Q67: Two moles of hydrogen gas would be
Q80: Cyclic compounds do not undergo halogenation reactions.
Q81: The branch name for a benzene group
Q84: The alkynes belong to an extensive family
Q87: Benzene is an aromatic hydrocarbon while cyclohexane
Q87: Consider the diagram below.Both materials would be
Q90: Aromatic compounds dissolve well in a nonpolar
Q92: Comparing unbranched alkanes and alkenes of the
Q93: As the number of double bonds increases
Q94: Another name for 1,2-dimethylbenzene is m -dimethylbenzene.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents