Carboxylic acids dissociate in a manner shown in the following equation.Identify the conjugate base for the acid of the forward reaction. 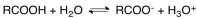
A) RCOOH
B) RCOO-
C) H2O
D) H3O+
Correct Answer:
Verified
Q54: Which of the following has the greatest
Q55: The following ion could be converted to
Q56: How many mL of a 0.150 M
Q58: Which of the following sets of reactants
Q59: If an intermediate in metabolism is a
Q62: Carboxylic acids are not polar and are
Q64: Butyric acid is composed of a molecule
Q67: Esters contain a carbonyl group attached directly
Q70: Pure liquid carboxylic acids are strongly hydrogen
Q75: An ester is equivalent to the inorganic
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents