A gas expands from A to B as shown in the graph.Calculate the work (in joules) done by the gas.(1 atm= 1.01 × 105 N/m2. ) 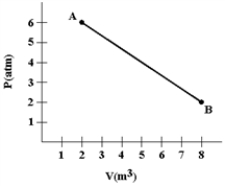
A) 12
B) 24
C) 1.21 × 106
D) 2.42 × 106
E) 3.64 × 106
Correct Answer:
Verified
Q23: In which process will the internal energy
Q24: For an astronaut working outside a spaceship,
Q25: Water at room temperature, 20°C, is pumped
Q26: A gas expands as shown in the
Q28: One gram of water is heated from
Q29: Five moles of an ideal gas expands
Q32: If an object feels cold to the
Q33: Determine the work done by 5 moles
Q36: Two kilograms of water at 100°C is
Q37: In an isothermal process
A) the volume remains
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents