The relation PV = nRT holds for all ideal gases.The additional relation PVγ holds for an adiabatic process.The figure below shows two curves: one is an adiabat and one is an isotherm.Each starts at the same pressure and volume.Which statement is correct? (Note: "∝" means "is proportional to". ) 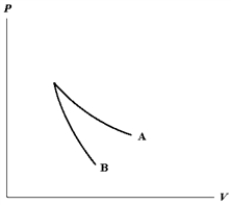
A) Isotherm: 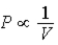 ;Adiabat:
;Adiabat: 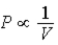 : A is both an isotherm and an adiabat.
: A is both an isotherm and an adiabat.
B) Isotherm: 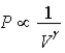 ;Adiabat:
;Adiabat: 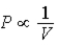 : B is an isotherm,A is an adiabat.
: B is an isotherm,A is an adiabat.
C) Isotherm: 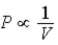 ;Adiabat:
;Adiabat: 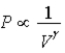 : A is an isotherm,B is an adiabat.
: A is an isotherm,B is an adiabat.
D) Isotherm: 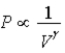 ;Adiabat:
;Adiabat: 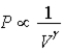 : B is both an isotherm and an adiabat.
: B is both an isotherm and an adiabat.
E) cannot answer without additional information about the starting temperature.
Correct Answer:
Verified
Q1: When we say that the speed of
Q3: An ideal gas is allowed to expand
Q5: A molecule in a uniform ideal gas
Q6: The average translational speed of a nitrogen
Q7: Suppose a box contains about 5.0 ×
Q9: A container having a volume of 1.0
Q10: Assume 3.0 moles of a diatomic gas
Q12: The air in an automobile engine at
Q16: Nitrogen gas is heated by a pulsed
Q20: During an adiabatic compression, a volume of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents