The theorem of equipartition of energy states that the energy each degree of freedom contributes to each molecule in the system (an ideal gas) is
A) 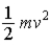 .
.
B) 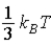 .
.
C) 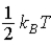 .
.
D) 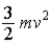 .
.
E) 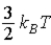 .
.
Correct Answer:
Verified
Q8: Five gas molecules are found to have
Q14: The average kinetic energy of a nitrogen
Q15: The internal energy of n moles of
Q16: The average molecular translational kinetic energy of
Q17: Find the specific heat (in cal/mole K)
Q19: Assume molecules have an average diameter of
Q24: The molar specific heat at constant pressure
Q25: The specific heat of an ideal gas
Q33: The root mean square speed of a
Q34: Air expands adiabatically (no heat in, no
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents