If n moles of an ideal gas are compressed isothermally from an initial volume V1 to a final volume V2,the change in entropy is
A) nR ln (V2/V1)
B) nRT ln (V2/V1)
C) nkB ln (V2/V1)
D) 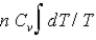
E) n Cv/T
Correct Answer:
Verified
Q21: 100 grams of molten lead (600°C) is
Q23: All real engines are less efficient than
Q24: Find the change in entropy (in J/K)
Q25: An ideal gas is allowed to undergo
Q31: Ten kilograms of water at 0°C is
Q33: Since Lice = 333 J/g, the change
Q34: Determine the change in entropy (in J/K)
Q36: Find the change in entropy (in J/K)
Q39: By operating a reversible heat engine with
Q40: The change in entropy when 1 kg
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents