What value of Z (atomic number) and A (mass number) result in the following β-decay? 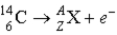
A) Z = 5;A = 14
B) Z = 4;A = 10
C) Z = 6;A = 14
D) Z = 7;A = 14
E) Z = 7;A = 13
Correct Answer:
Verified
Q2: The isotope, tritium, has a half-life of
Q4: How many radioactive atoms are present in
Q6: Find the ratio of the binding energy
Q7: Find the binding energy per nucleon (in
Q11: 44 g of petrified wood was found
Q12: What value of Z (atomic number)and A
Q12: The ratio of the radius of a
Q14: An alpha particle is emitted from a
Q15: The ratio of the density of a
Q17: For large mass number nuclei which are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents