Which of the following molecules should have the same molecular shape and approximate bond angles as ammonia, 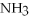 ?
?
A) BH3
B) CH4
C) SO3
D) PH3
E) NO2
Correct Answer:
Verified
Q101: List the following bonds in order of
Q109: If an ionic bond is stronger than
Q110: What is happening at the molecular level
Q111: Water, H2O, and methane, CH4, have about
Q112: Which of the following molecules would you
Q113: Which of the above substances would have
Q114: Which of the following is the strongest?
A)A
Q115: A substance consisting of which molecule shown
Q118: Which of the following molecules is the
Q124: Which of the following intermolecular forces best
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents