Two chemical structures are shown, one of a typical gasoline molecule and the other of a typical motor oil molecule. Which is which? 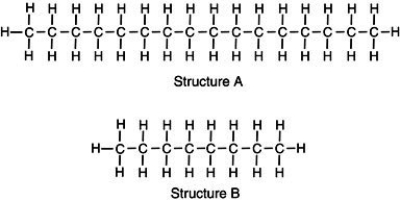 Base your reasoning not on memorization but rather upon what you know about molecular interactions and the various physical properties of gasoline and motor oil.
Base your reasoning not on memorization but rather upon what you know about molecular interactions and the various physical properties of gasoline and motor oil.
A) Structure A represents the gas molecule because there are more bonds to gain energy from, giving it a higher energy content than oil.
B) Structure A represents motor oil, illustrating a molecule with greater induced dipole-induced dipole molecular interactions thus, the molecules are strongly attracted to one another.
C) Structure B represents the oil molecule. Because oil molecules are smaller, they can compact closer together, giving the appearance of a thicker solution than gasoline.
D) Structure B represents crude oil which is processed to generate longer molecules of gasoline to prevent toxic vapors from harming consumers.
Correct Answer:
Verified
Q129: Which of the following intermolecular forces best
Q131: Which of the following intermolecular forces best
Q138: Given the following diagram, describe what happens
Q139: Why is calcium fluoride, CaF2, a high
Q141: Chlorine, Cl2, is a gas at room
Q142: Why is the surface area of a
Q143: Plastic wrap is made of nonpolar molecules
Q144: How are oxygen molecules attracted to water
Q145: The boiling point of 1,4-butanediol is 230°C.
Q146: Consider the boiling points of the following
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents