Account for the observation that ethyl alcohol, C2H5OH, dissolves readily in water but dimethyl ether, CH3OCH3, which has the same number and kinds of atoms, does not. 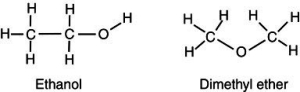
A) The hydrogens on the dimethyl ether surround the molecule, shielding the inner atoms from interacting with the water.
B) Because the carbons arrange themselves in a straight line, the ethanol can interact more easily with more water molecules, thus increasing its solubility.
C) The high electronegativity of the carbon-oxygen-carbon bond on dimethyl ether creates a strong dipole charge on the ends of the molecule, making it highly soluble in water.
D) Because dimethyl ether lacks an -OH group, it is significantly less polar than is ethyl alcohol and is not readily soluble in water.
Correct Answer:
Verified
Q86: Would you expect to find more dissolved
Q88: What is the main difference between a
Q91: At 10°C, which is more concentrated-a saturated
Q93: Which of the following might best describe
Q94: Why might a solvent like turpentine be
Q95: When you set a pot of tap
Q98: Phosphate ions, PO43-, were once added to
Q100: Which of the following statements does not
Q100: Fish don't live very long in water
Q104: What happens if you were to place
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents