Jewelry is often manufactured by electroplating an expensive metal such as gold over a cheaper metal. A setup for this process can be sketched as follows: 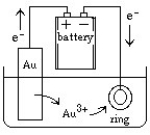 What would happen if the battery connections were suddenly reversed?
What would happen if the battery connections were suddenly reversed?
A) The ring would continue to electroplate with gold.
B) Gold ions in solution would get reduced and settle to the bottom of the container.
C) Gold ions in solution would begin to electroplate onto the gold electrode.
D) All electrolysis would stop.
Correct Answer:
Verified
Q146: Sodium metal is
A)oxidized in the production of
Q163: Water is 88.88 percent oxygen by mass.
Q164: Copper atoms have a greater tendency to
Q165: A chemical equation for the combustion of
Q169: The oxidation of iron to rust is
Q172: Rust has a tendency to form when
Q173: Why are combustion reactions generally exothermic?
A)Hydrogen easily
Q173: How many electrons are transferred from iron
Q174: Iron atoms have a greater tendency to
Q177: Why is oxygen usually an oxidizing agent?
A)It
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents