If the average kinetic energy of the molecules of a monatomic ideal gas at 55°C is doubled,what is the resulting temperature of the gas?
A) It becomes 55× 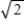 °C.
°C.
B) It becomes 656°C.
C) It becomes 110°C.
D) It becomes 383°C.
Correct Answer:
Verified
Q84: If the temperature of a volume of
Q85: The noble gases,listed by increasing molecular weight,are
Q86: Consider two containers with the same volume
Q87: Evaporation cools the liquid that is left
Q88: What is the internal energy of 50
Q90: What is the root-mean-square speed of chlorine
Q91: The internal energy of a monatomic ideal
Q92: The temperature of a quantity of ideal
Q93: A tank with a volume of 0.15
Q94: John rapidly pulls a plunger out of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents