Sample #1 is made from an isotope with decay constant 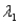
And sample #2 is made from an isotope with decay constant 
,where 
) Which of the following statements must be true?
A) The activity of sample #2 is greater than that of sample #1.
B) The activity of sample #1 is greater than that of sample #2.
C) The half-life exhibited for sample #1 is greater than that of sample #2.
D) The half-life exhibited for sample #2 is greater than that for sample #1
Correct Answer:
Verified
Q41: Tritium (3H)has a half-life of 12.3 years
Q42: The alpha emission process results in the
Q43: The neutron is radioactive and can beta
Q44: The beta emission process results in the
Q45: Uranium-238 decays to Thorium-234 by emitting which
Q47: A radioactive isotope that emits an alpha
Q48: The original nucleus and the final nucleus
Q49: The half-life of 18N is 0.62 s.What
Q50: What particle is emitted when 240Pu decays
Q51: About what fraction of the initial activity
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents