An archeologist discovers the bones of a person believed to have been dead for 600 years.Using carbon dating,they wish to confirm the time of the person's demise.The rate of change of 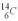 is 0.270 Bq/g of carbon for the bones of a person who had just died.If the archeologist is correct about the person's time of death,what rate of change of
is 0.270 Bq/g of carbon for the bones of a person who had just died.If the archeologist is correct about the person's time of death,what rate of change of 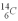 should they find when they carbon date the bones? The half life of
should they find when they carbon date the bones? The half life of 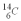 is 5.73 x 103 y.
is 5.73 x 103 y.
A) 0.245 Bq/g
B) 0.267 Bq/g
C) 0.251 Bq/g
D) 0.238 Bq/g
E) 0.259 Bq/g
Correct Answer:
Verified
Q2: A radioactive atom undergoes
Q3: A certain radioactive isotope decays to one
Q4: A radioactive atom undergoes
Q5: A certain radioactive isotope decays to one
Q6: A drug containing Q8: The rate of change of Q9: A radioactive atom emits an alpha particle.When![]()

Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents