An electron in the hydrogen atom makes a transition from the 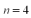 state to an nth state.The electron gains 0.50 eV of energy.What value of
state to an nth state.The electron gains 0.50 eV of energy.What value of 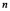 is the final state of the electron?
is the final state of the electron?
A) 0
B) 2
C) 4
D) 6
E) 8
Correct Answer:
Verified
Q12: Positronium consists of a bound state of
Q13: The Bohr model of hydrogen is based
Q14: The minimum orbital angular momentum quantum number
Q15: The frequency of the Q16: The electron in a singly ionized helium Q18: The period of rotation of electron on Q19: The maximum orbital angular momentum quantum number Q20: In the Bohr model of hydrogen,as the Q21: A group of hydrogen-like atoms (atoms with Q22: Find the wavelength of light emitted by![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents