A hydrogen atom is in a quantum state with principal quantum number n = 3.The atom emits a photon with a wavelength of 660 nm.Determine the maximum possible orbital angular momentum of the electron after emission.
A) 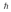
B) 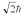
C) 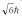
D) 
E) 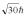
Correct Answer:
Verified
Q24: What is the longest possible wavelength that
Q25: The muon has the same charge
Q26: The Pfund series results from emission/absorption of
Q27: For an electron in the ground state
Q28: The frequency of rotation of electron on
Q30: A group of hydrogen-like atoms (atoms with
Q31: In a "muonic" atom an electron is
Q32: The Pfund series results from emission/absorption of
Q33: A group of hydrogen-like atoms (atoms with
Q34: A hydrogen atom is in a quantum
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents