What are the quantum numbers n and l for a hydrogen atom with E = - (13.6/16) eV and L = 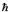 ?
?
A) n = 4,l = 2
B) n = 4,l = 6
C) n = 3,l = 3
D) n = 3,l = 6
Correct Answer:
Verified
Q53: A photon of wavelength 155 nm is
Q54: Identify the element that has the configuration
Q55: Krypton has 36 electrons.What is the expected
Q56: The radial probability density of a hydrogen
Q57: A 5.0 UV laser emits light that
Q59: In a system where the energy difference
Q60: A photon of wavelength 1.17
Q61: What is the shortest wavelength photon that
Q62: About how many photons are emitted per
Q63: How many electrons are allowed to inhabit
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents