An ideal gas 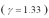 with a pressure of 1.00 atm at room temperature (300 K) is compressed from 500.0 cm3 to a final volume.The compression is adiabatic and the final pressure of the gas is 25.1 atm.What final volume of the gas?
with a pressure of 1.00 atm at room temperature (300 K) is compressed from 500.0 cm3 to a final volume.The compression is adiabatic and the final pressure of the gas is 25.1 atm.What final volume of the gas?
A) 25.2 cm3
B) 31.5 cm3
C) 44.3 cm3
D) 52.1 cm3
E) 58.4 cm3
Correct Answer:
Verified
Q10: For an ideal gas,the temperature measured in
Q11: An ideal gas is initially at a
Q12: A cylinder containing nitrogen gas is at
Q13: Two moles of an ideal gas
Q14: When is the root-mean-squared speed of a
Q16: A cylinder containing 5.00 mol of nitrogen
Q17: Three moles of an ideal gas
Q18: If 0.100 mol of an ideal monatomic
Q19: In a period of 6.00 s,7.00 *
Q20: A cylinder containing 5.00 mol of nitrogen
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents