An ideal gas 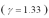 with a pressure of 1.00 atm at room temperature (300 K) is compressed from 500.0 cm3 to a final volume of 58.4 cm3.The compression is adiabatic.What final pressure of the gas?
with a pressure of 1.00 atm at room temperature (300 K) is compressed from 500.0 cm3 to a final volume of 58.4 cm3.The compression is adiabatic.What final pressure of the gas?
A) 21.0 atm
B) 25.1 atm
C) 17.4 atm
D) 8.56 atm
E) 13.2 atm
Correct Answer:
Verified
Q1: The specific heat of an ideal gas
Q2: An ideal gas Q4: For an ideal gas,the temperature measured in Q5: A cylinder containing nitrogen gas is at Q6: In a period of 6.00 s,6.00 * Q7: A cylinder containing 5.00 mol of nitrogen Q8: 15.0 liters of an ideal monatomic gas Q9: A cylinder containing 5.00 mol of nitrogen Q10: For an ideal gas,the temperature measured in Q11: An ideal gas is initially at a![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents