Two containers contain the same gas at different temperatures and pressures as detailed in the drawing.The small container has a volume of 1 liter and the large container has a volume of 2 liters.The two containers are then connected to each other using a thin tube and the system is allowed to reach equal pressure and temperature in both containers.If the final temperature is 300 K,what is the final pressure? You may assume that the connecting tube has negligible volume and mass. 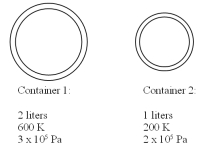
A) 400 kPa
B) 250 kPa
C) 200 kPa
D) 300 kPa
Correct Answer:
Verified
Q54: A cylinder with a piston contains 2.00
Q55: Nuclear fusion is achieved by fusing hydrogen
Q56: Two identical containers hold equal masses of
Q57: A cylinder with a piston contains 2.00
Q58: The diameter of an oxygen molecule
Q60: The diameter of an oxygen molecule is
Q61: The pressure of an ideal gas is
Q62: One hundred grams of liquid argon is
Q63: There are two vessels;one contains twice as
Q64: A scuba diver at a depth of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents