Two containers contain the same gas at different temperatures and pressures as detailed in the drawing below.The small container has a volume of 1 liter and the large container has a volume of 2 liters.The two containers are then connected to each other using a thin tube and the system is allowed to reach equal pressure and temperature in both containers.If the final pressure is 200 kPa,what is the final temperature? You may assume that the connecting tube has negligible volume and mass. 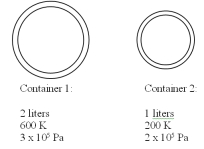
A) 400 K
B) 500 K
C) 550 K
D) 300 K
Correct Answer:
Verified
Q45: A cylinder with a piston contains 2.00
Q46: The specific heat of steam (M =
Q47: At standard atmospheric pressure,at what temperature is
Q48: Uranium has two isotopes U238 and U235.Enriched
Q49: A gas expands at constant pressure
Q51: Helium gas is filled in a
Q52: Nuclear fusion is achieved by fusing hydrogen
Q53: A gas is in a closed container
Q54: A cylinder with a piston contains 2.00
Q55: Nuclear fusion is achieved by fusing hydrogen
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents