For a class demonstration,your physics instructor pours 1.00 kg of steam at 100 C over 5.00 kg of ice at 0.00 C,allows the system to reach equilibrium and then he is going to measure the temperature of the system.While the system used in the class demonstration reaches equilibrium,you are given the latent heats of ice and steam and the specific heat of water to be,respectively: 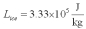 ;
; 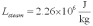 ;
; 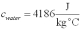 and you are asked to calculate the final equilibrium temperature of the system.What value did you find?
and you are asked to calculate the final equilibrium temperature of the system.What value did you find?
A) 22.5 C
B) 40.4 C
C) 64.3 C
D) 80.3 C
E) 95.8 C
Correct Answer:
Verified
Q2: A 2.0-kg piece of steel with
Q3: A copper sheet of area
Q4: How many joules of energy need
Q5: A gas has an initial volume of
Q6: A copper sheet of area
Q7: When the volume of a thermodynamic system
Q8: On which large surface should you place
Q9: A gas has an initial volume of
Q10: A 2.00-g lead bullet is shot
Q11: The pressure of a dilute gas as
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents