If a neutral element has the following chemical symbol, how many electrons does it have? 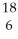 O
O
A) 6
B) 18
C) 12
D) 24
E) none of the above
Correct Answer:
Verified
Q28: If a neutral element has the following
Q32: You could swallow a capsule of germanium,Ge
Q34: If an element has 18 protons and
Q40: If an element has 10 protons and
Q47: If a neutral element has 8 neutrons
Q49: Why did Rutherford propose a model for
Q50: If an element has 9 protons and
Q50: Isotopes of the same element have a
Q54: Boron has primarily two isotopes, one with
Q58: If an element has 15 protons and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents