Consider the various frequencies of the three photons emitted from the following three individual electron transitions in the figure below: n=3 to n=2; n=2 to n=1; n=3 to n=1. These transitions would produce three spectral lines in a spectroscope. If the energy spacing between the levels were equal, would this affect the number of spectral lines? 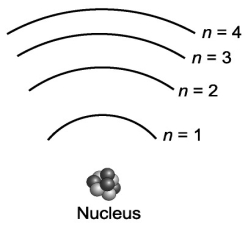
A) Yes, two otherwise separate lines would converge into a single more intense line.
B) No, but the spacing between the spectral lines would change.
C) Yes, two otherwise separate lines would converge into a single less intense line.
D) No, but some would then require a prism in order to be seen.
Correct Answer:
Verified
Q105: What property of an electron makes it
Q110: How might you distinguish a sodium-vapor street
Q118: Light is emitted as an electron transitions
Q119: Which of the following statements about electrons
Q121: Neon, Ne, (number 10)has a relatively large
Q122: From which of the following atoms would
Q122: How many electrons are there in the
Q131: Which of the following atoms is the
Q132: Which of the following might best explain
Q137: Some older cars vibrate loudly when driving
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents