Which would you expect to have a higher melting point: sodium chloride, NaCl, or aluminum oxide, 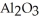 ?
?
A) The aluminum oxide has a higher melting point because it is a larger molecule and has a greater number of molecular interactions.
B) NaCl has a higher melting point because it is a solid at room temperature.
C) The aluminum oxide has a higher melting point because of the greater charges of the ions, and hence the greater force of attractions between them.
D) The aluminum oxide has a higher melting point because of the covalent bonds within the molecule.
Correct Answer:
Verified
Q49: Is an ionic compound an example of
Q63: Which of the following describes the reason
Q66: Which of the following describes the reason
Q68: Which of the following describes the reason
Q69: What property of alloys make them ideal
Q73: Why are metal ores so valuable?
A)They are
Q74: Which are closer together: the two nuclei
Q76: Which of the following describes the reason
Q80: Why is it better to recycle metals
Q88: Many analogies can be drawn between chemical
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents