Which of the following molecules has two pairs of nonbonding electrons on the central atom?
A) 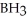
B) 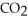
C) 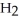 O
O
D) 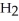 S
S
E) C and D
Correct Answer:
Verified
Q81: Which of the following bonds would be
Q106: The acronym VSEPR stands for _.
A)valence shell
Q113: In two dimensions sulfuric acid, 
Q114: What is the molecular shape of the
Q115: How many electrons are used to draw
Q117: How many substituents are on the following
Q119: How many electrons are used to draw
Q120: Phosphine is a covalent compound that contains
Q134: Which of the following compounds would be
Q140: When is the geometry of a molecule
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents