Consider the electron-dot geometries of P 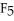 and S
and S 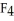 . Which do you expect is the more polar compound?
. Which do you expect is the more polar compound?
A) The P  should be more polar because it has a greater number of fluorines.
should be more polar because it has a greater number of fluorines.
B) The P 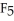 should be more polar because it is more symmetrical.
should be more polar because it is more symmetrical.
C) The S 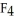 should be more polar because it has a lone pair of electrons.
should be more polar because it has a lone pair of electrons.
D) The S  should be more polar because it is less symmetrical.
should be more polar because it is less symmetrical.
Correct Answer:
Verified
Q82: Which of the following molecules would contain
Q99: Which of the above substances would have
Q126: True or False: The more shells in
Q133: Which of the following molecules contains a
Q136: The source of an atom's electronegativity is
Q137: Polar molecules tend to be _.
A)symmetrical
B)elongated
C)asymmetrical
D)diatomic
Q139: Which of the following molecules is polar?
Q141: Ammonia, N Q142: An individual carbon-oxygen bond is polar. Yet Q143: Water, ![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents