Under which of the following conditions would you expect the highest solubility of oxygen gas in water?
A) high temperature and low 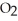 pressure above the solution
pressure above the solution
B) low temperature and high 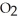 pressure above the solution
pressure above the solution
C) low temperature and low 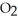 pressure above the solution
pressure above the solution
D) high temperature and high 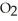 pressure above the solution
pressure above the solution
E) The 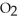 solubility is independent of temperature and pressure.
solubility is independent of temperature and pressure.
Correct Answer:
Verified
Q68: Which of the following might have the
Q70: What happens when the molecule-to-molecule attractions in
Q76: How is the solubility of a gas
Q83: A solid has a solubility at room
Q86: Would you expect to find more dissolved
Q88: How can you tell whether a sugar
Q89: If the solubility of a compound is
Q91: Suggest why sodium chloride,NaCl,is insoluble in gasoline.Consider
Q95: When you set a pot of tap
Q115: A saturated solution of compound X in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents