Like water, hydrogen fluoride, HF, and ammonia, N 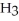 , have relatively high boiling points. Explain.
, have relatively high boiling points. Explain.
A) Even though HF and N 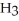 have ionic bonds, like water they have a strong attraction for themselves.
have ionic bonds, like water they have a strong attraction for themselves.
B) Since these are all relatively small molecules, they can compact more tightly together, and will require more energy to be separated from each other.
C) The polar molecules of each of these materials have relatively strong attractions for themselves, which translates to relatively high boiling points.
D) Since these molecules interact with each other in long chains, they have many regions of attraction and are held together relatively tightly, thus, they are harder to pull apart when boiling.
Correct Answer:
Verified
Q83: If an evaporating liquid cools, then does
Q84: Will wrapping a bottle in a wet
Q85: Water coming out of volcanic sea vents
Q94: Which of the following statements best describes
Q95: A material with a high specific heat
Q96: Water has a low specific heat because
Q100: Does putting a lid over a pot
Q101: If water had a lower specific heat,
Q102: The specific heat of a substance is
Q106: Given the following specific heats and that
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents