What is the mass of a water molecule, 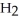 O, in grams? (1 amu = 1.661 × 10-24 g)
O, in grams? (1 amu = 1.661 × 10-24 g)
A) 18 grams
B) 1.661 × 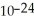 grams
grams
C) 2.99 × 10-23 grams
D) none of the above
Correct Answer:
Verified
Q43: What is the number of grams of
Q50: What is the number of molecules of
Q63: Three identical candles are placed in the
Q64: Two candles of the same mass, one
Q65: Which has more atoms: 64.058 g of
Q66: Which has more atoms: 17.031 g of
Q69: What is the mass of an oxygen
Q70: Which has the greater mass, 1.204 ×
Q71: What is the mass of a water
Q72: A 1.00 carat pure diamond has a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents