For each of the following unbalanced equations, which is depicted: oxidation or reduction? a) Cr → 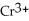 b) Sn →
b) Sn → 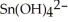
A) a) oxidation; b) reduction
B) a) reduction; b) oxidation
C) a) reduction; b) reduction
D) a) oxidation; b) oxidation
Correct Answer:
Verified
Q35: The general chemical equation for photosynthesis is
Q37: What element is behaves as the oxidizing
Q38: Iron atoms, Fe, are better reducing agents
Q41: What is the purpose of the salt
Q42: Chemical equations need to be balanced not
Q101: How does an atom's electronegativity relate to
Q123: Which of the following statements best describes
Q127: Which of the following describes an electrode?
A)Any
Q133: Clorox is a laundry bleaching agent used
Q139: Which of the following statements best describes
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents