The general chemical equation for photosynthesis is shown below. Through this reaction, are the oxygens in the water molecules, H2O, oxidized or reduced? 6 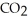 + 6
+ 6  O →
O → 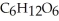 + 6
+ 6 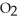
A) The oxygens of the water molecules are oxidized.
B) The oxygens of the water molecules are reduced.
C) The oxygens of some of these water molecules are oxidized while others are reduced.
D) The oxygens of the water molecules are neither oxidized nor reduced.
Correct Answer:
Verified
Q23: When lightning strikes, nitrogen molecules, 
Q24: Based on their relative positions in the
Q28: What correlation might you expect between an
Q28: Consider the following molecules. What is the
Q29: Chemical equations need to be balanced not
Q30: Hydrogen sulfide, H2S, burns in the presence
Q30: What might the relationship be between an
Q35: What might the relationship be between an
Q111: Q120: ![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents