Why is 2,4,5-trifluorophenol much more acidic than phenol? 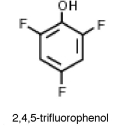
A) The fluorines adjacent to the OH group are large and shield the hydroxl group from making the solution basic.
B) The fluorines de-stabilize the benzene ring and thus give the structure more acidic characteristics.
C) The fluorines have high electronegativities and help to stabilize the negative charges, which makes it even easier for the hydrogen to be lost.
D) False! 2,4,5-trifluorophenol is actually not more acidic than phenol.
Correct Answer:
Verified
Q44: How does ingested methanol lead to the
Q58: Why do ethers have lower boiling points
Q63: Which of the following commonly acts as
Q87: Why is a phenol more acidic than
Q90: Which region (a-b-c)is a hydroxyl functional group?
A)a
B)b
C)c
D)b
Q91: The formula for a certain ether is
Q92: Which region (a-b-c)is a phenolic functional group?
A)a
B)b
C)c
D)b
Q94: Ethanol can be made industrially from two
Q97: One of the skin-irritating components of poison
Q100: Which of the following compounds is an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents