Explain why caprylic acid, C  (C
(C 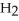
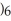 COOH, dissolves in a 5 percent aqueous solution of sodium hydroxide but caprylaldehyde, C
COOH, dissolves in a 5 percent aqueous solution of sodium hydroxide but caprylaldehyde, C 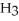 (C
(C 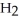
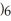 CHO, does not.
CHO, does not.
A) With two oxygens the caprylic acid is about twice as polar the caprylaldehyde.
B) The caprylaldehyde is a gas at room temperature.
C) The caprylaldehyde behaves as a reducing agent, which neutralizes the sodium hydroxide.
D) The caprylic acid reacts to form the water soluble salt.
Correct Answer:
Verified
Q73: Suggest an explanation for why aspirin has
Q96: What is the main difference between High
Q123: The fungus causing Dutch Elm disease is
Q128: A common approach to building a complex
Q129: An amino acid is an organic molecule
Q131: Which of the following molecules contains a
Q134: The following reaction is used to make
Q135: Which of the monomers below would be
Q135: What happens when you use a monomer
Q139: An organic chemist's retrosynthesis analysis focuses on
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents