Camphor is a 10-carbon odoriferous natural product made from the joining of two isoprene units plus the addition of a ketone. Shown below on the left is its chemical structure. On the right is shown the camphor structure with two particular chemical "units" circled. What is/are the name(s) of these two units? 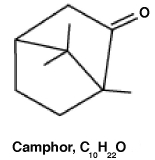
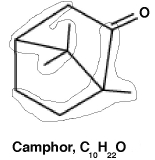
A) Both chemical units are isoprene.
B) Both chemical units are styrene.
C) Both chemical units are isopropene.
D) The upper chemical unit is isopropene and the lower unit is styrene.
Correct Answer:
Verified
Q86: The compound 6-aminohexanoic acid is used to
Q94: What type of polymer would be best
Q142: Which of the following polymers has the
Q152: What was a significant reason for the
Q153: Why is rubber heated with sulfur more
Q154: Which of the following polymers would you
Q156: What property of nitrocellulose was exploited in
Q157: Besides synthetic rubber, which of the following
Q160: Which of the following polymers has similar
Q162: Why are Ping-Pong balls so highly flammable?
A)They
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents