A cyclic process is carried out on an ideal gas such that it returns to its initial state at the end of a cycle, as shown in the pV diagram in the figure. If the process is carried out in a counter-clockwise sense around the enclosed area, as shown on the figure, then the magnitude of the enclosed area represents 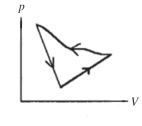
A) the heat that flows out of the gas.
B) the work done on the gas.
C) the heat added to the gas.
D) the work done by the gas.
Correct Answer:
Verified
Q2: A certain gas is compressed adiabatically.The amount
Q7: Which one of the following is a
Q8: Which of the following is a false
Q12: An ideal gas undergoes an isothermal expansion.During
Q17: When water at 0°C freezes,the entropy of
Q19: If the efficiency of a Carnot engine
Q20: An important feature of the Carnot cycle
Q24: The temperature changes from 35°F during the
Q28: At what,if any,temperature are the numerical readings
Q39: A temperature change of 20°C corresponds to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents