An ideal gas undergoes the process a→b→c→a shown in the pV diagram. In the figure, Pa = Pc = 240 kPa, Vb = Vc = 40 L, Va = 15 L, and Pb = 400 kPa. How much heat is gained by the gas in this a→b→c→a process? 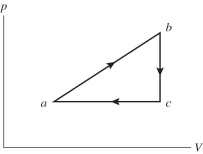
A) 1000 J
B) 1500 J
C) 2000 J
D) 2500 J
E) 3000 J
Correct Answer:
Verified
Q32: A certain heat engine extracts 1.30 kJ
Q33: A heat engine with an efficiency of
Q33: A heat engine has an efficiency of
Q52: An expansion process on an ideal
Q53: A fluid in an insulated, flexible
Q55: A compression, at a constant pressure
Q59: A cylinder contains 1.50 moles of an
Q60: A sealed rigid tank contains 29
Q61: The figure shows a pV diagram for
Q62: An ideal Carnot engine extracts 529
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents