The figure shows part of the energy level diagram of a certain atom. The energy spacing between levels 1 and 2 is twice that between 2 and 3. If an electron makes a transition from level 3 to level 2, the radiation of wavelength λ is emitted. What possible radiation wavelengths might be produced by other transitions between the three energy levels? 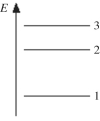
A) both λ/2 and λ/3
B) only λ/2
C) both 2λ and 3λ
D) only 2λ
Correct Answer:
Verified
Q3: The orbital angular momentum quantum number ℓ
Q6: The Balmer series is formed by electron
Q8: To which of the following values of
Q10: According to the quantum mechanical model of
Q12: According to Pauli's exclusion principle,how many electrons
Q17: If a hydrogen atom originally in a
Q18: A hydrogen atom is in the 6h
Q20: The Paschen series is formed by electron
Q21: The magnetic quantum number m1 can have
Q32: What is the wavelength in the Balmer
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents