The Lewis structure of acetate ion is as follows: 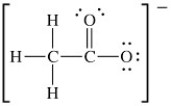 What is the formal charge on oxygen atom that contains three lone pairs of electrons?
What is the formal charge on oxygen atom that contains three lone pairs of electrons?
Correct Answer:
Verified
Q102: In the best Lewis structure for CN-,what
Q104: How many lone pairs of electrons are
Q106: How many lone pairs are on the
Q130: Which element can act as the centre
Q134: Which of the following compounds is hypercoordinate?
A)
Q145: Draw the Lewis dot structure for Al3+.
Q150: Describe a covalent bond.
Q154: Define dipole moment.
Q163: Describe the difference between a pure covalent
Q166: Define formal charge.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents