Consider the molecule below.Determine the molecular geometry at each of the two labelled carbons. 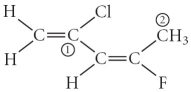
A) C1 = tetrahedral,C2 = linear
B) C1 = trigonal planar,C2 = bent
C) C1 = bent,C2 = trigonal planar
D) C1 = trigonal planar,C2 = tetrahedral
E) C1 = trigonal pyramidal,C2 = seesaw
Correct Answer:
Verified
Q3: Determine the electron geometry (eg)and molecular geometry
Q8: Determine the electron geometry (eg)and molecular geometry
Q23: Determine the electron geometry for the molecule
Q26: Determine the molecular geometry for the molecule
Q30: Determine the electron geometry for the molecule
Q32: Give the approximate bond angle for a
Q33: Determine the electron geometry (eg)and molecular geometry
Q33: Determine the electron geometry (eg)and molecular geometry
Q37: Determine the electron geometry for the molecule
Q39: Determine the molecular geometry for the molecule
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents