Consider the molecule below.Determine the hybridization at each of the two labelled carbons. 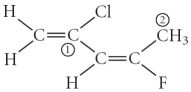
A) C1 = sp3,C2 = sp3d
B) C1 = sp,C2 = sp2
C) C1 = sp2,C2 = sp3d
D) C1 = sp3d,C2 = sp3d2
E) C1 = sp2,C2 = sp3
Correct Answer:
Verified
Q56: Consider the following compound.How many sigma and
Q78: How many of the following molecules contain
Q96: Draw the Lewis structure for N2H2. What
Q100: Draw the Lewis structure for COCl2. What
Q100: Draw the Lewis structure for
Q101: Use the molecular orbital diagram shown to
Q103: Use the molecular orbital diagram shown to
Q104: Use the molecular orbital diagram shown to
Q110: Draw a molecular orbital diagram and use
Q119: List the number of sigma bonds and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents